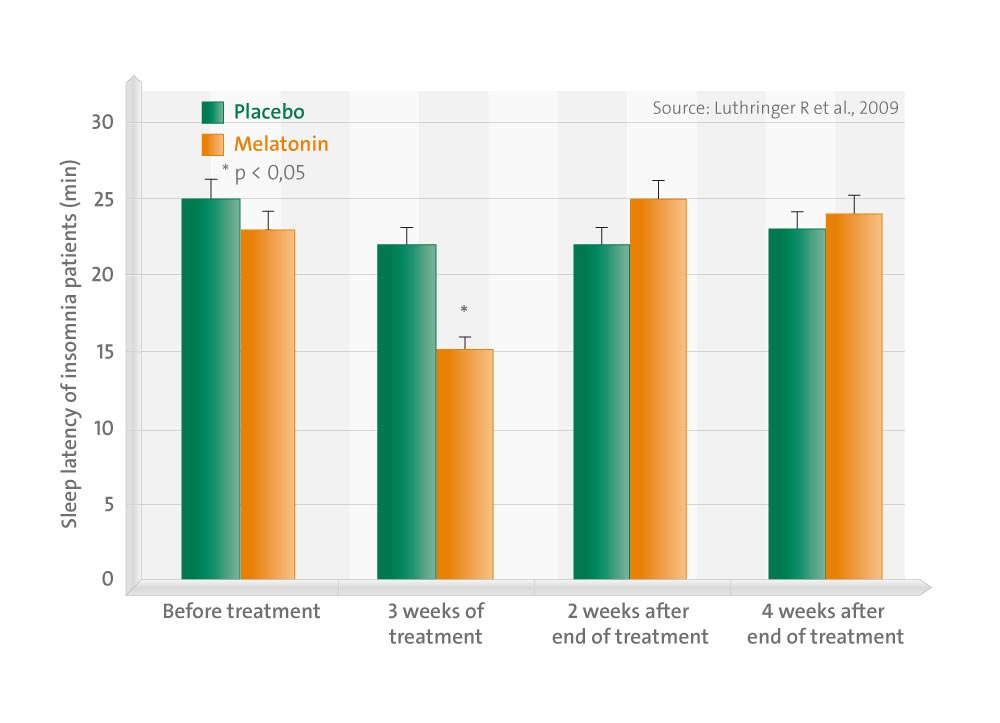
Objectives of this study were to investigate the effects of prolonged-release melatonin 2 mg (PRM) on sleep and subsequent daytime psychomotor performance in patients aged > or =55 years with primary insomnia, as defined by fourth revision of the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association.
Patients (N = 40) were treated nightly single-blind with placebo (2 weeks), randomized double-blind to PRM or placebo (3 weeks) followed by withdrawal period (3 weeks). Sleep was assessed by polysomnography, all-night sleep electroencephalography spectral analysis and questionnaires. Psychomotor performance was assessed by the Leeds Psychomotor Test battery. By the end of the double-blind treatment, the PRM group had significantly shorter sleep onset latency (9 min; P = 0.02) compared with the placebo group and scored significantly better in the Critical Flicker Fusion Test (P = 0.008) without negatively affecting sleep structure and architecture. Half of the patients reported substantial improvement in sleep quality at home with PRM compared with 15% with placebo (P = 0.018). No rebound effects were observed during withdrawal. In conclusion, nightly treatment with PRM effectively induced sleep and improved perceived quality of sleep in patients with primary insomnia aged > or =55 years. Daytime psychomotor performance was not impaired and was consistently better with PRM compared with placebo. PRM was well tolerated with no evidence of rebound effects.