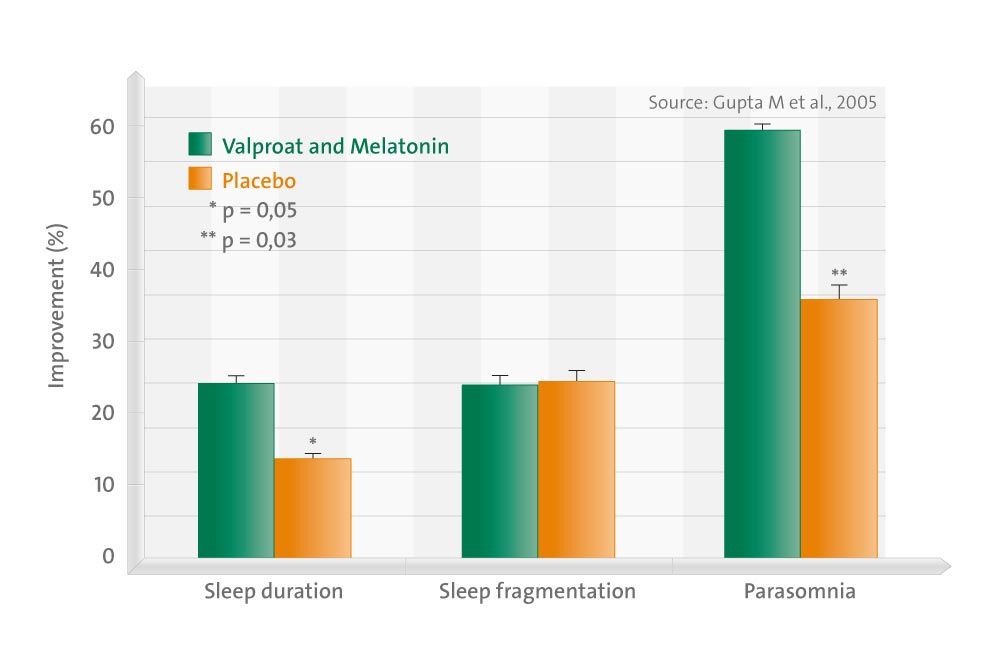
This double-blind, randomized, placebo-controlled study in epileptic children, aged 3 to 12 years, evaluated the effect of add-on melatonin on the sleep behavior of these children on sodium valproate monotherapy using a parental questionnaire.
Of the 31 patients, 16 randomly received add-on melatonin, whereas 15 received add-on placebo. The questionnaire showed good internal consistency in our patient population (Cronbach’s alpha = .83). The percentage decrease in the median total sleep score was 24.4 (range 0.0-34.9) in the valproate + melatonin group compared with 14.0 (range -2.2-18.8) in the valproate + placebo group, the difference being statistically significant (P < .05). The median percentage decrease in the parasomnias score was 60 (range 0.0-70.8) in the valproate + melatonin group compared with 36.4 (range 0.0-63.2) in the valproate + placebo group, the difference being statistically significant (P < .05). There was no significant difference between the percentage decrease in the daytime drowsiness scores and sleep fragmentation scores. Parent-child interaction subscale scores were not significantly different between age groups. The age at onset of seizures and the type of seizures did not correlate significantly to the total sleep scores. Given that sleep problems are known to complicate epilepsy, add-on melatonin, which has a wide safety window, can be of promise in the pharmacotherapy of pediatric epilepsy.