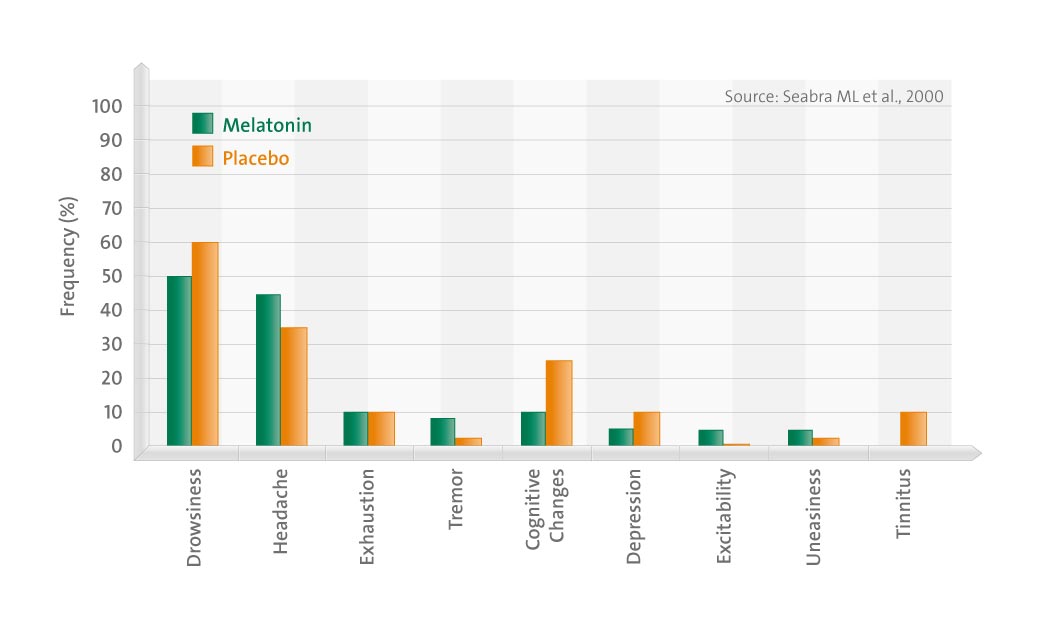
The objective of the present study was to assess the toxicology of melatonin (10 mg), administered for 28 days to 40 volunteers randomly assigned to groups receiving either melatonin (N = 30) or placebo (N = 10) in a double-blind fashion.
The following measurements were performed: polysomnography (PSG), laboratory examinations, including complete blood count, urinalysis, sodium, potassium and calcium levels, total protein levels, albumin, blood glucose, triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL), urea, creatinine, uric acid, glutamic-oxalacetic transaminase (GOT), glutamic-pyruvate transaminase (GPT), bilirubin, alkaline phosphatase, gama-glutamic transaminase (GGT), T3, T4, TSH, LH/FSH, cortisol, and melatonin serum concentrations.
In addition, the Epworth Somnolence Scale (ESS) and a sleep diary (SD) were also applied to the volunteers 1 wk before each PSG. In addition, the volunteers were asked about possible side effects (SE) that appeared during the treatment.
The study was carried out according to the following timetable:
- Visit 0, filling out the term of consent and inclusion criteria;
- Visit 1, PSG, laboratory examinations, ESS, SD, melatonin serum concentrations;
- Visit 2, SD, melatonin serum concentrations, SE;
- Visit 3, melatonin serum concentrations, PSG, ESS, SE;
- Visit 4, laboratory examinations, SE, melatonin serum concentrations, SD;
- Visit 5, PSG, ESS, SE.
Analysis of the PSG showed a statistically significant reduction of stage 1 of sleep in the melatonin group.
No other differences between the placebo and melatonin groups were obtained. In the present study we did not observe, according to the parameters analyzed, any toxicological effect that might compromise the use of melatonin at a dose of 10 mg for the period of time utilized in this study.