Cadmium (Cd) is a heavy metal affecting human health both through environmental and occupational exposure. There is evidence that Cd accumulates in several organs and is carcinogenic to humans.
In vivo, Cd mimics the effect of estrogens in the uterus and mammary gland. In estrogen-responsive breast cancer cell lines, Cd stimulates proliferation and can also activate the estrogen receptor independent of estradiol. The ability of this metalloestrogen to increase gene expression in MCF7 cells is blocked by anti-estrogens suggesting that the activity of these compounds is mediated by ER alpha.
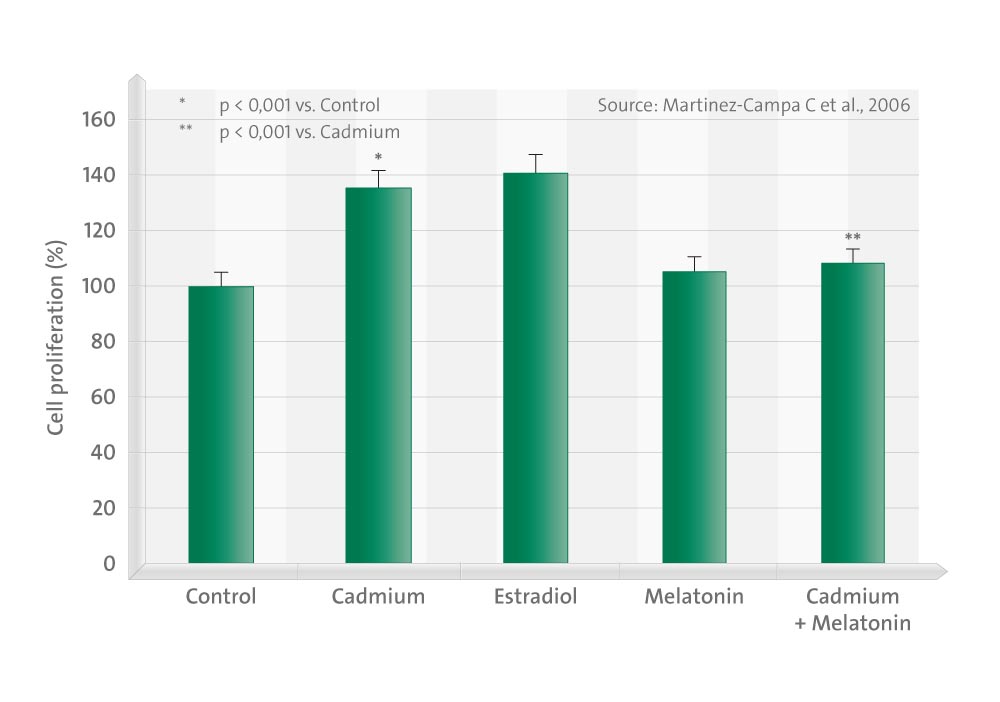
The aims of this work were to test whether melatonin inhibits Cd-induced proliferation in MCF7 cells, and also to study whether melatonin specifically inhibits Cd-induced ER alpha transactivation. We show that melatonin prevents the Cd-induced growth of synchronized MCF7 breast cancer cells. In transient transfection experiments, we prove that both ER alpha- and ER beta-mediated transcription are stimulated by Cd.
Melatonin is a specific inhibitor of Cd-induced ER alpha-mediated transcription in both estrogen response elements (ERE)- and AP1-containing promoters, whereas ER beta-mediated transcription is not inhibited by the pineal indole. Moreover, the mutant ER alpha-(K302G, K303G), unable to bind calmodulin, is activated by Cd but becomes insensitive to melatonin treatment.
These results proved that melatonin inhibits MCF7 cell growth induced by Cd and abolishes the stimulatory effect of the heavy metal in cells expressing ER alpha at both ERE-luc and AP1-luc sites. We can infer from these experiments that melatonin regulates Cd-induced transcription in both ERE- and AP1 pathways. These results also reinforce the hypothesis of the anti-estrogenic properties of melatonin as a valuable tool in breast cancer therapies.