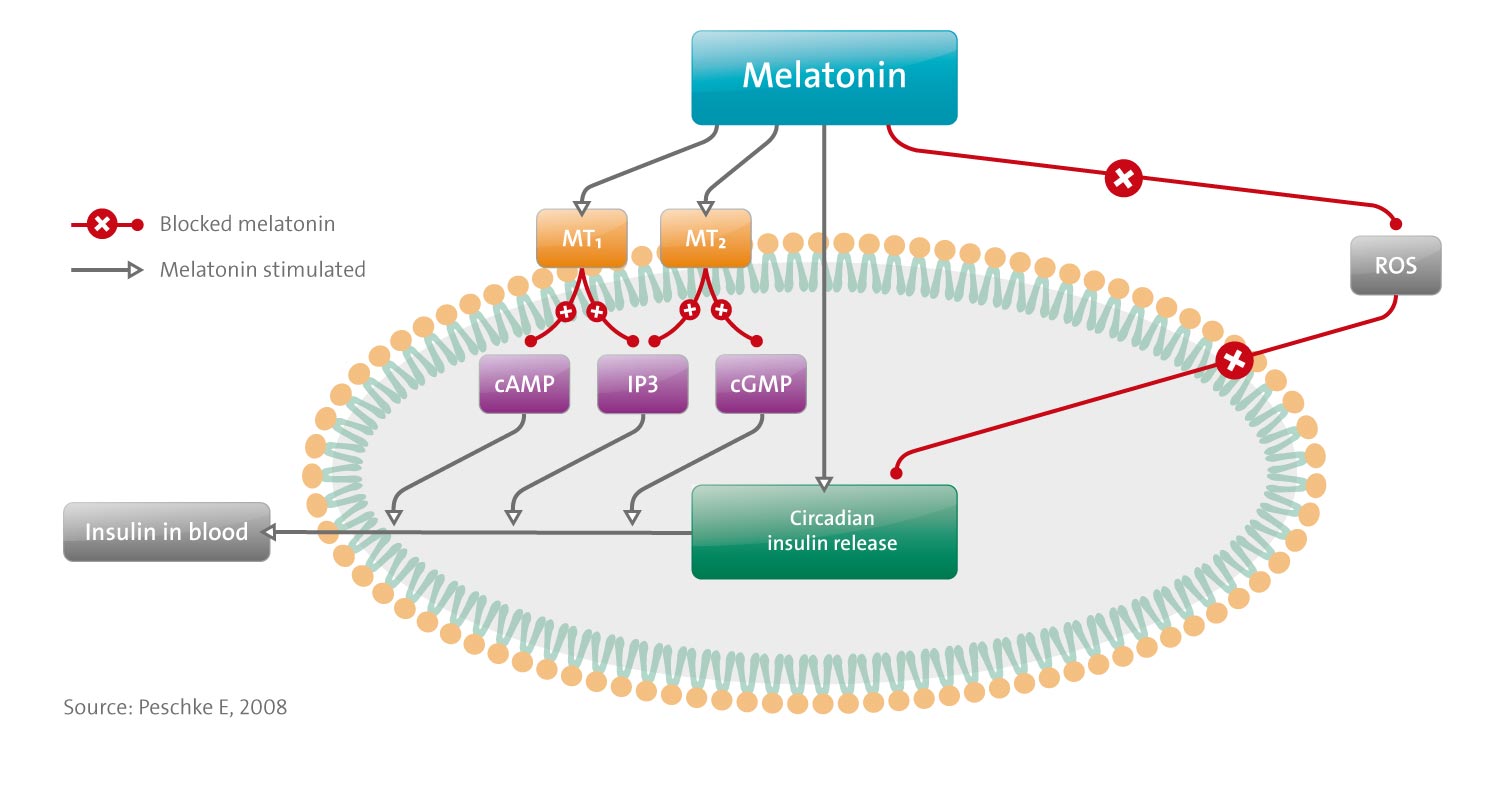
Melatonin influences insulin secretion both in vivo and in vitro.
- The effects are MT(1)-and MT(2)-receptor-mediated.
- They are specific, high-affinity, pertussis-toxin-sensitive, G(i)-protein-coupled, leading to inhibition of the cAMP-pathway and decrease of insulin release. [Correction added after online publication 4 December 2007: in the preceding sentence, ‘increase of insulin release’ was changed to ‘decrease of insulin release’.] Furthermore, melatonin inhibits the cGMP-pathway, possibly mediated by MT(2) receptors. In this way, melatonin likely inhibits insulin release. A third system, the IP(3)-pathway, is mediated by G(q)-proteins, phospholipase C and IP(3), which mobilize Ca(2+) from intracellular stores, with a resultant increase in insulin.
- Insulin secretion in vivo, as well as from isolated islets, exhibits a circadian rhythm. This rhythm, which is apparently generated within the islets, is influenced by melatonin, which induces a phase shift in insulin secretion.
- Observation of the circadian expression of clock genes in the pancreas could possibly be an indication of the generation of circadian rhythms in the pancreatic islets themselves.
- Melatonin influences diabetes and associated metabolic disturbances. The diabetogens, alloxan and streptozotocin, lead to selective destruction of beta-cells through their accumulation in these cells, where they induce the generation of ROS. Beta-cells are very susceptible to oxidative stress because they possess only low-antioxidative capacity. Results suggest that melatonin in pharmacological doses provides protection against ROS.
- Finally, melatonin levels in plasma, as well as the arylalkylamine-N-acetyltransferase (AANAT) activity, are lower in diabetic than in nondiabetic rats and humans. In contrast, in the pineal gland, the AANAT mRNA is increased and the insulin receptor mRNA is decreased, which indicates a close interrelationship between insulin and melatonin.