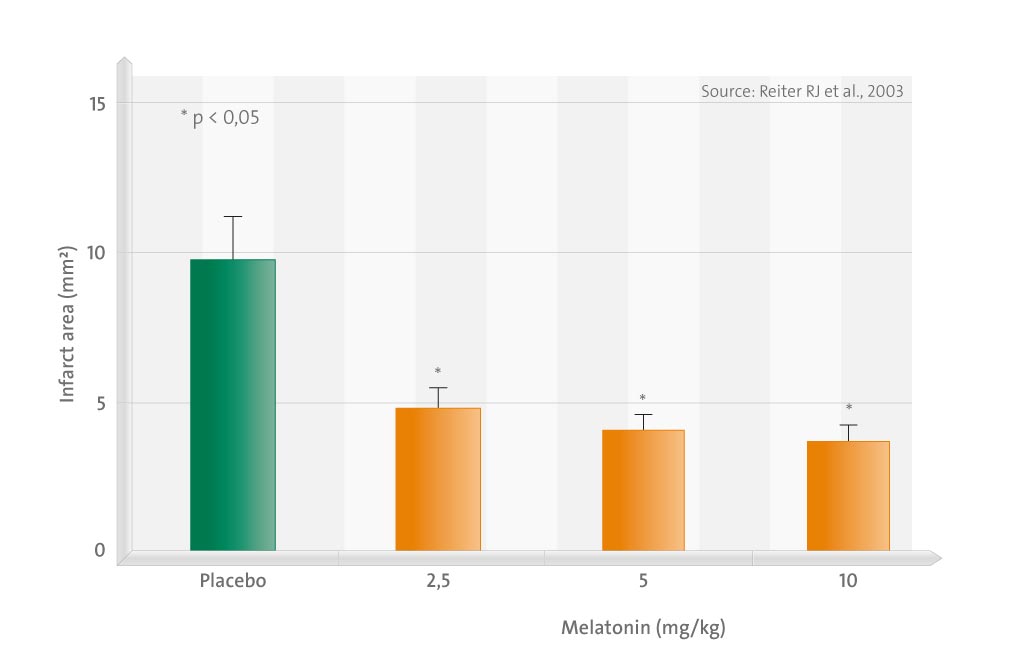
This review summarizes the numerous reports that have documented the neuroprotective actions of melatonin in experimental models of ischemia/reperfusion injury (stroke).
In these investigations, which have used three species (rat, gerbil, and cat), melatonin was universally found to reduce brain damage that normally occurs as a consequence of the temporary interruption of blood flow followed by the reflow of oxygenated blood to the brain. The exogenous administration of melatonin in these experimental stroke models reduced infarct volume, lowered the frequency of apoptosis, increased the number of surviving neurons, reduced reactive gliosis, lowered the oxidation of neural lipids and oxidatively damaged DNA, induced bcl-2 gene expression (the activity of which improves cell survival), upregulated excision repair cross-complementing factor 6 (an essential gene for preferential DNA excision repair), restrained poly(ADP ribose) synthetase (which depletes cellular NAD resulting in the loss of ATP) activity, and improved neurophysiologic outcomes.
Under no circumstances did melatonin exacerbate the damage associated with ischemia/reperfusion injury. As well as the beneficial pharmacologic actions of melatonin, several studies show that a relative deficiency of endogenous melatonin exaggerates neural damage due to stroke; this suggests that even physiologic concentrations of melatonin normally serve to protect the brain against damage. The primary action to explain melatonin’s protective effects may relate to its ubiquitous direct and indirect antioxidative actions, although other beneficial functions of melatonin are not precluded.