Background
Benzodiazepines are the most frequently used drug for the treatment of insomnia. Prolonged use of benzodiazepine therapy is not recommended. However, many patients, particularly older patients, have difficulties discontinuing therapy. Melatonin, a hormone that is produced at night by the pineal gland, promotes normal sleep in humans and augments sleep induction by benzodiazepine therapy.
Objective
To assess whether the administration of melatonin could facilitate the discontinuation of benzodiazepine therapy in patients with insomnia.
Methods
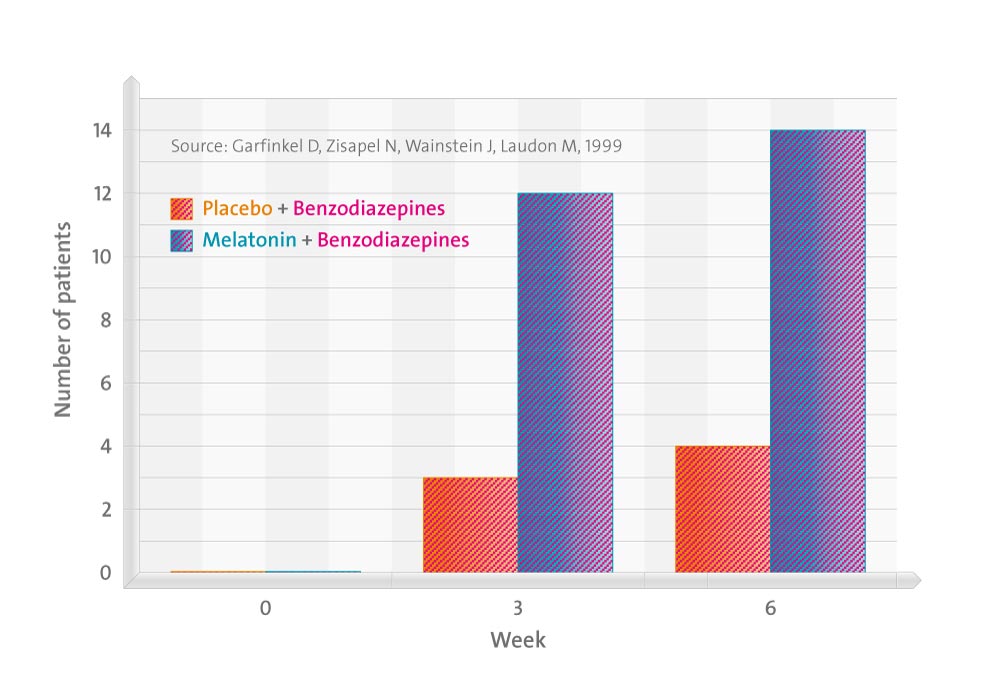
Thirty-four subjects receiving benzodiazepine therapy were enrolled in the 2-period study. In period 1, patients received (double-blinded) melatonin (2 mg in a controlled-release formulation) or a placebo nightly for 6 weeks. They were encouraged to reduce their benzodiazepine dosage 50% during week 2, 75% during weeks 3 and 4, and to discontinue benzodiazepine therapy completely during weeks 5 and 6. In period 2, melatonin was administered (single-blinded) for 6 weeks to all subjects and attempts to discontinue benzodiazepine therapy were resumed. Benzodiazepine consumption and subjective sleep-quality scores were reported daily by all patients. All subjects were then allowed to continue melatonin therapy and follow-up reassessments were performed 6 months later.
Results
By the end of period 1, 14 of 18 subjects who had received melatonin therapy, but only 4 of 16 in the placebo group, discontinued benzodiazepine therapy (P = .006). Sleep-quality scores were significantly higher in the melatonin therapy group (P = .04). Six additional subjects in the placebo group discontinued benzodiazepine therapy when given melatonin in period 2. The 6-month follow-up assessments revealed that of the 24 patients who discontinued benzodiazepine and received melatonin therapy, 19 maintained good sleep quality.
Conclusion
Controlled-release melatonin may effectively facilitate discontinuation of benzodiazepine therapy while maintaining good sleep quality.